Which of the Following Are Not Decomposition Reactions
Decomposition is a kind of reaction where a single substance breaks into two or more products. Decomposition of ozone O 3 to oxygen O 2 is exothermic.

Decomposition Reaction Definition Examples Applications
2 CH4 3 O2 2 CO2 4 H2O a.

. If the reaction was conducted with solid zinc twice and crushedpowdered zinc once. Decomposition reaction - Separation of a substance into two or more substances that may differ from each other and from the original substance. A decomposition reaction is a type of chemical reaction in which a single compound breaks down into two or more elements or new compoundsIt is a reaction in which two substances react with each other to make a single substance.
2Mg O2 2MgO. If your measurements of an object are 1008g 925g and 550g and the actual mass is 1175g are your measurements accurate precise both or neither. Correct option is D.
CaO H2O CaOH2. Many important biological reactions are freely reversible. A KCIO3 KCI O2 B 2 Na Cl2 2NaCl C Mg HCl H2 MgCl2 D HCI NaOH.
C aC O3 C aO C O2 C a C O 3 C a O C O 2. C Cd NO32 Na2S CdS 2 NaNO3. QUESTION 21 Which of the following reactions is not a decomposition reaction.
The correct statement is only reaction C is correct. Which of the following reactions is a decomposition reaction. Water is a reactant.
B energy in the form of heat or light is often produced. Which of the following equations is not a double replacement reaction. The following are not decomposition reactions.
Magnesium ribbon is burnt in air. N a O H H C l N a C l H 2 O is a displacement reaction. Which of the following is NOT a decomposition reaction.
Dear student The reaction given in the first option is an example of electrical decomposition or electrolysis. Recomposition 2 See answers Willchemistry Willchemistry Choice D Recomposition Recomposition is not one of the five general. What are the Applications of Decomposition Reactions.
Methane and carbon dioxide are products. A AgNO3 NaCl AgCl NaNO3 B H2SO4 MgOH2 H2O MgSO4 C AlCl3 H2O AlOH3 HCl D Mg. Which of the following statements is not true about the reaction.
A synthesis reaction that produces a molecule of water is called hydrolysis At equilibrium a synthesis reaction and a decomposition reaction occur at a balanced rate. The decomposition reaction is a chemical reaction shows the decomposition of a compound into its constituent elements or compounds. Therefore H2 Cl2 ----2HCl is a COMBINATION REACTION.
No not all decomposition reactions are endothermic. Exchange reactions involve both synthesis and decomposition. C the reactants are usually a metal and a nonmetal.
Which one of the following is not true concerning automotive air bags A They are inflated as a result of a decomposition reaction B they are loadedwith sodium aside initially C the gas used for inflating them is oxygen D the two products of the decomposition reaction are sodium and nitrogen E a gas is produced when the air bag activates. Which of the following is an example of a combination reaction. The reaction illustrated in this diagram would be.
Hence it is a decomposition reaction. Which of the following is not one of the five general types of reactions. 1 mark aThermal b Electrical c Displacement dPhoto.
A the reactants are generally two ionic compounds in aqueous solution. Which of the following reactions is a decomposition reaction. Muxakara and 1 more users found.
However the latter is more common than the former. A HCl NaOH NaCl H2O B Mg HCl H2 MgCl2 C KClO3 KCl O2 D CaCl2 H2O CaCl2 2H2O. KCIO3 KCI O2.
More than one response is correct. CaO H2O CaOH2. You should answer in terms of particles.
As in a decomposition reaction a single reactant breaks down to form two products but in this we see that H2Cl2--- 2HCl is a combination reaction and NOT a decomposition reaction. Decomposition reaction is defined as the reaction in which one large compound breaks down into smaller compounds. Asked 3 days ago in Chemistry by Shwetapandey 120k points Which of the following is not a decomposition reaction.
Explain why the rate of reaction changes as the concentration of the acid the reactor increases. D 2 Mg O2 2 MgO. The decomposition of NO to N 2 O 2 is exothermic.
2HgOs 2H90 O2g H2g Cl2g 2HClg NH4NO3s N2Og 2H2Og MgOH2s MgOs H2Og 2H2O2aq - 2H200 O2g ОО. 1 Decomposition reactions and addition reactions are two terms that describe the same type of reaction. NaOH is a base known as sodium hydroxide.
A decomposition reaction can be both endothermic or exothermic. So options ABC are decomposition reaction. This problem has been solved.
Which of the following is an oxidizing agent. A HCI NaOH NaCl H2O B Mg HCl H2 MgCl2 C CaCl2 H2O CaCl2 2H2O D KCIO3 KCI O2 7. A Conversion of starch into glucose follow decomposition reaction because there is a breaking of a starch molecule into glucose.
Which of the following is not the type of a decomposition chemical reaction. B Calcium carbonate on heating breaks down into calcium oxide and carbon dioxide. B NH4Cl NH3 HCl.
Disgestion of food in body. More than one response is correct. A 2 N2 3 H2 2 NH3.
C In a decomposition reaction. The decomposition reaction for sodium hydroxide follow the equation. C Magnesium ribbon when burnt in air it gets oxidized to form magnesium oxide by.
By Stoichiometry of the reaction. 2K C I O3 2K C I 3O2 2 K C I O 3 2 K C I 3 O 2. Characterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true-false status of the statements using the choices.
A NH4NO2 s N2g H2O1 b H2O2aq H2Ol O2g C KCls Ks. Which of the following reactions is a decomposition reaction.

Which Of The Following Are Not Decomposition Reactions Brainly Com
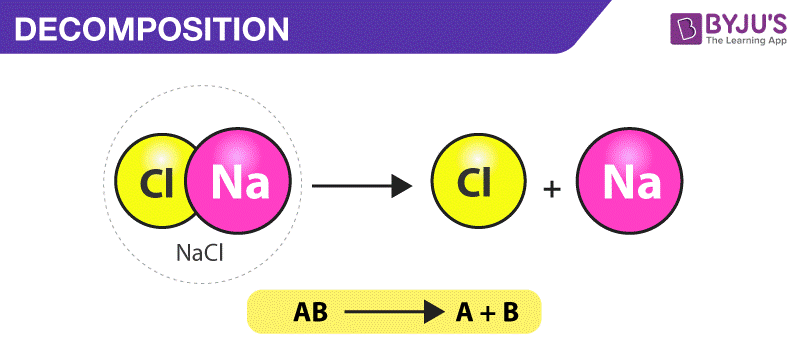
Decomposition Reaction Definition Types Examples Uses

Chemical Reactions 4 Of 11 Decomposition Reactions An Explanation Youtube
Belum ada Komentar untuk "Which of the Following Are Not Decomposition Reactions"
Posting Komentar